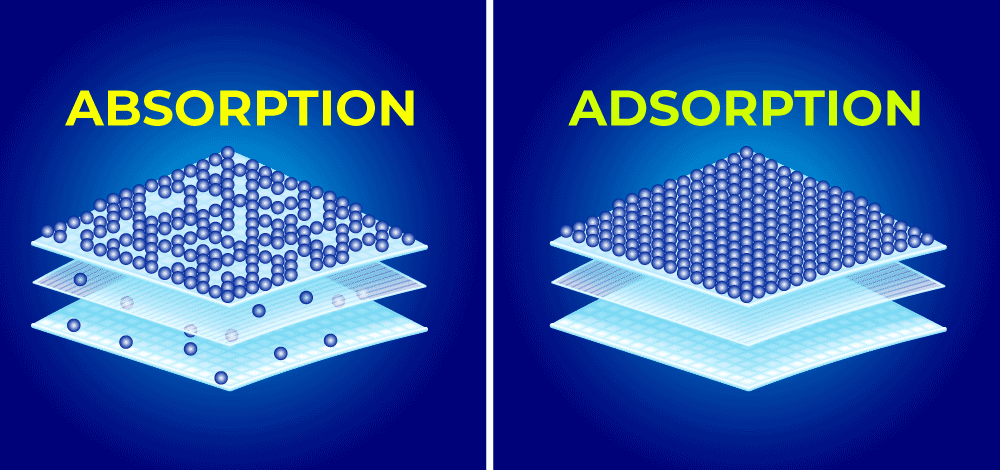
Chemistry students and professionals often encounter two seemingly similar processes that play crucial roles in countless applications: adsorption and absorption. While these terms sound nearly identical and are frequently confused, they represent fundamentally different phenomena with distinct mechanisms, characteristics, and applications.
Understanding the difference between adsorption and absorption isn’t just academic—it’s essential knowledge for anyone working in fields ranging from environmental science to chemical engineering, materials science to biotechnology. Whether you’re designing water purification systems, developing new materials, or optimizing industrial processes, grasping these concepts will enhance your ability to solve real-world problems effectively.
This comprehensive guide will explore both processes in detail, examining their underlying mechanisms, key differences, and practical applications. We’ll also address common misconceptions and provide insights into the latest innovations that leverage these phenomena for environmental and industrial solutions.
Understanding Adsorption: A Surface Chemistry Phenomenon
Adsorption definition: Adsorption is the process by which atoms, ions, or molecules from a gas, liquid, or dissolved solid accumulate on the surface of another material. This surface chemistry phenomenon results in higher concentrations of the substance at the interface compared to the bulk phase.
In adsorption, three key components interact:
- Adsorbate: The substance being adsorbed (accumulated on the surface)
- Adsorbent: The material providing the surface where adsorption occurs
- Interface: The boundary where the two phases meet
The Molecular Mechanism of Adsorption
At the molecular level, adsorption occurs when molecules in the fluid phase encounter a solid surface and experience attractive forces. These forces can be physical (van der Waals forces) or chemical (covalent bonding), leading to two distinct types:
Physical Adsorption (Physisorption):
- Involves weak van der Waals forces
- Reversible at moderate temperatures
- Multiple molecular layers can form
- Low heat of adsorption (typically 5-40 kJ/mol)
Chemical Adsorption (Chemisorption):
- Involves chemical bond formation
- Usually irreversible or requires high temperatures for desorption
- Typically forms monolayers
- High heat of adsorption (typically 40-400 kJ/mol)
Surface Area: The Critical Factor
The effectiveness of adsorption depends heavily on surface area. Materials with high surface areas, such as activated carbon (500-3000 m²/g), silica gel (300-800 m²/g), and zeolites (300-900 m²/g), serve as excellent adsorbents. This relationship explains why powdered materials often outperform their granular counterparts in adsorption applications.
Real-World Examples of Adsorption
Activated Carbon Water Filters: These systems demonstrate adsorption’s power in water purification. Activated carbon’s extensive pore structure provides enormous surface area, allowing it to adsorb chlorine, organic compounds, and various contaminants. The process is so effective that a single gram of activated carbon can have a surface area equivalent to several football fields.
Silica Gel Packets: Those small packets found in electronics packaging use silica gel’s exceptional ability to adsorb moisture from air. The silica gel doesn’t absorb water into its bulk structure but rather accumulates water molecules on its extensive internal surface area.
Gas Masks: Military and industrial gas masks rely on activated carbon and specialized adsorbents to remove toxic gases from breathable air. Different adsorbent materials target specific contaminants—some for organic vapors, others for acidic gases or mercury vapor.
Understanding Absorption: A Bulk Phenomenon
Absorption definition: Absorption is the process by which one substance is uniformly distributed throughout the volume of another substance. Unlike adsorption, this bulk phenomenon involves the penetration of molecules throughout the entire structure of the absorbing material.
The Mechanism of Absorption
During absorption, molecules of the absorbed substance (absorbate) penetrate into the absorbing material (absorbent) and distribute evenly throughout its volume. This process doesn’t depend primarily on surface area but rather on the material’s capacity to accommodate foreign molecules within its structure.
The driving force for absorption is typically a concentration gradient, with molecules moving from regions of high concentration to regions of low concentration until equilibrium is reached. This process follows Fick’s laws of diffusion and can be described mathematically using differential equations that account for concentration gradients and diffusion coefficients.
Key Characteristics of Absorption
- Uniform Distribution: The absorbed substance distributes evenly throughout the absorbing material
- Concentration Independence: The concentration remains constant throughout the material at equilibrium
- Constant Rate: The absorption rate typically remains steady throughout the process
- Volume Dependency: The process depends on the volume and internal structure of the absorbent
Real-World Examples of Absorption
Sponge Absorbing Water: When a dry sponge contacts water, the water molecules penetrate the sponge’s porous structure and distribute throughout its volume. The water doesn’t just coat the surface—it fills the internal spaces, demonstrating true absorption.
Drying Agents in Chemical Processes: Calcium chloride and other hygroscopic materials absorb water vapor from air by incorporating water molecules into their crystalline structure. The water becomes part of the material’s bulk composition, forming hydrated compounds.
Ink Blotter Paper: Traditional ink blotters demonstrate absorption’s uniform distribution characteristic. The paper’s fibers absorb excess ink, drawing it away from the writing surface and distributing it throughout the paper’s thickness.
Key Differences Between Adsorption and Absorption
Understanding the fundamental differences between these processes is crucial for selecting appropriate materials and designing effective systems:
Surface vs. Bulk Phenomenon
- Adsorption: Occurs exclusively at the interface between phases
- Absorption: Occurs throughout the entire volume of the absorbing material
Concentration Variations
- Adsorption: Creates concentration differences between surface and bulk
- Absorption: Maintains uniform concentration throughout the material
Rate Characteristics
- Adsorption: Exhibits high initial rates that decrease as equilibrium approaches
- Absorption: Maintains relatively constant rates throughout the process
Temperature Dependency
- Adsorption: Generally decreases with increasing temperature (for physical adsorption)
- Absorption: May increase or decrease with temperature depending on the specific system
Capacity Limitations
- Adsorption: Limited by available surface area and number of active sites
- Absorption: Limited by the material’s total volume and structural capacity
Industrial and Environmental Applications
Adsorption Applications
Water Treatment: Municipal water treatment facilities use granular activated carbon to remove organic contaminants, taste, and odor compounds. Advanced systems employ different adsorbent materials in series to target specific contaminants.
Air Purification: Industrial air cleaning systems use zeolites, activated carbon, and specialized adsorbents to remove volatile organic compounds (VOCs), sulfur compounds, and other air pollutants.
Catalysis: Many industrial catalysts work through adsorption mechanisms, where reactants adsorb onto catalyst surfaces, react, and then desorb as products. This process is fundamental to petroleum refining, chemical synthesis, and emissions control.
Chromatography: This analytical and preparative technique separates compounds based on their different adsorption affinities for stationary phases. Gas chromatography and liquid chromatography rely entirely on selective adsorption and desorption processes.
Absorption Applications
Chemical Processing: Absorption towers in chemical plants use liquid absorbents to remove specific compounds from gas streams. For example, ammonia production facilities use water to absorb ammonia from gas mixtures.
Carbon Capture: Some carbon capture technologies use liquid solvents that absorb CO₂ from flue gases. The CO₂-rich solvent is then heated to release concentrated CO₂ for storage or utilization.
Pharmaceutical Manufacturing: Drug delivery systems often rely on absorption processes, where active ingredients are absorbed into carrier materials for controlled release.
Synergistic Applications: When Both Processes Work Together
Modern applications increasingly leverage both adsorption and absorption in complementary ways:
Hybrid Water Treatment Systems: Advanced water purification systems combine adsorption (for removing specific contaminants) with absorption (for general purification and pH adjustment).
Smart Materials: Innovative materials designed for environmental remediation may use surface adsorption for rapid contaminant capture followed by absorption for long-term storage and neutralization.
Energy Storage: Some battery technologies rely on adsorption at electrode surfaces combined with absorption of electrolyte ions into electrode materials.
Environmental Impact and Sustainability
Adsorption Technologies
Adsorption-based systems offer several ecological advantages:
- Regenerability: Many adsorbents can be regenerated through heating or chemical treatment
- Energy Efficiency: Physical adsorption typically requires less energy than chemical processes
- Selective Removal: Can target specific contaminants without affecting beneficial compounds
Absorption Technologies
Absorption processes contribute to sustainability through:
- Complete Utilization: Can achieve near-complete removal of target substances
- Integration Potential: Often integrate well with existing industrial processes
- Resource Recovery: Can enable recovery and reuse of valuable materials
Latest Innovations and Future Directions
Advanced Materials
Research continues to develop new materials that optimize both adsorption and absorption processes:
- Metal-Organic Frameworks (MOFs): Highly porous materials with tunable properties for specific applications
- Graphene-Based Adsorbents: Exceptional surface areas and selective adsorption capabilities
- Bio-Based Absorbents: Sustainable materials derived from agricultural waste and biomass
AI and Machine Learning Applications
Artificial intelligence increasingly optimizes adsorption and absorption processes:
- Predictive Modeling: AI algorithms predict optimal operating conditions for maximum efficiency
- Material Design: Machine learning accelerates the discovery of new adsorbent and absorbent materials
- Process Control: Real-time optimization of industrial systems using AI-driven control systems
Frequently Asked Questions
What is the key difference between adsorption and absorption?
The fundamental difference lies in where the process occurs: adsorption happens only at the surface interface between materials, while absorption occurs throughout the entire volume of the absorbing material.
Can a substance undergo both adsorption and absorption?
Yes, many systems exhibit both processes simultaneously. For example, when treating wastewater, contaminants may initially adsorb onto particle surfaces and subsequently absorb into the particle’s internal structure.
How does temperature affect adsorption and absorption processes?
For physical adsorption, increasing temperature typically decreases the extent of adsorption due to increased molecular motion. Absorption processes may increase or decrease with temperature depending on the specific materials and whether the process is endothermic or exothermic.
What are some common real-world applications of adsorption?
Common applications include water filtration using activated carbon, air purification systems, gas masks, catalytic converters in vehicles, and analytical techniques like chromatography.
How is adsorption used in environmental science?
Environmental applications include removing heavy metals from contaminated water, capturing volatile organic compounds from air, treating industrial wastewater, and remediation of contaminated soils using specialized adsorbent materials.
How is absorption used in industrial processes?
Industrial uses include chemical separation processes, purification of gases and liquids, manufacturing of composite materials, and various chemical reactions where one component must be uniformly distributed throughout another.
What is the role of surface area in adsorption?
Surface area is critical for adsorption because the process occurs only at interfaces. Materials with higher surface areas, such as activated carbon or zeolites, provide more sites for molecules to adsorb, resulting in greater capacity and efficiency.
How does the concentration gradient affect absorption?
Concentration gradients drive absorption processes, with molecules moving from high-concentration regions to low-concentration regions. The steeper the gradient, the faster the absorption rate until equilibrium is achieved.
What is desorption, and why is it important?
Desorption is the reverse of adsorption—the release of previously adsorbed molecules from a surface. It’s crucial for regenerating adsorbent materials, recovering valuable substances, and understanding the complete adsorption-desorption cycle in various applications.
Mastering These Fundamental Processes
The distinction between adsorption and absorption extends far beyond academic terminology. These processes form the foundation for countless technologies that address some of our most pressing challenges—from cleaning drinking water to capturing carbon emissions, from developing new materials to optimizing industrial processes.
For students and professionals alike, understanding these mechanisms provides the knowledge base needed to innovate and solve complex problems. Whether you’re designing next-generation water treatment systems, developing sustainable materials, or optimizing chemical processes, the principles of surface chemistry and bulk phenomena will guide your work.
As technology continues to advance, the applications of adsorption and absorption will only expand. By mastering these fundamental concepts, you’ll be equipped to contribute to solutions that benefit both industry and the environment, making you a more effective scientist, engineer, or technical professional.
The future belongs to those who understand how molecules behave at interfaces and within materials. Now you have the knowledge to be part of that future.